
Insights+: EMA Marketing Authorization of New Drugs in May 2024
Shots:
-
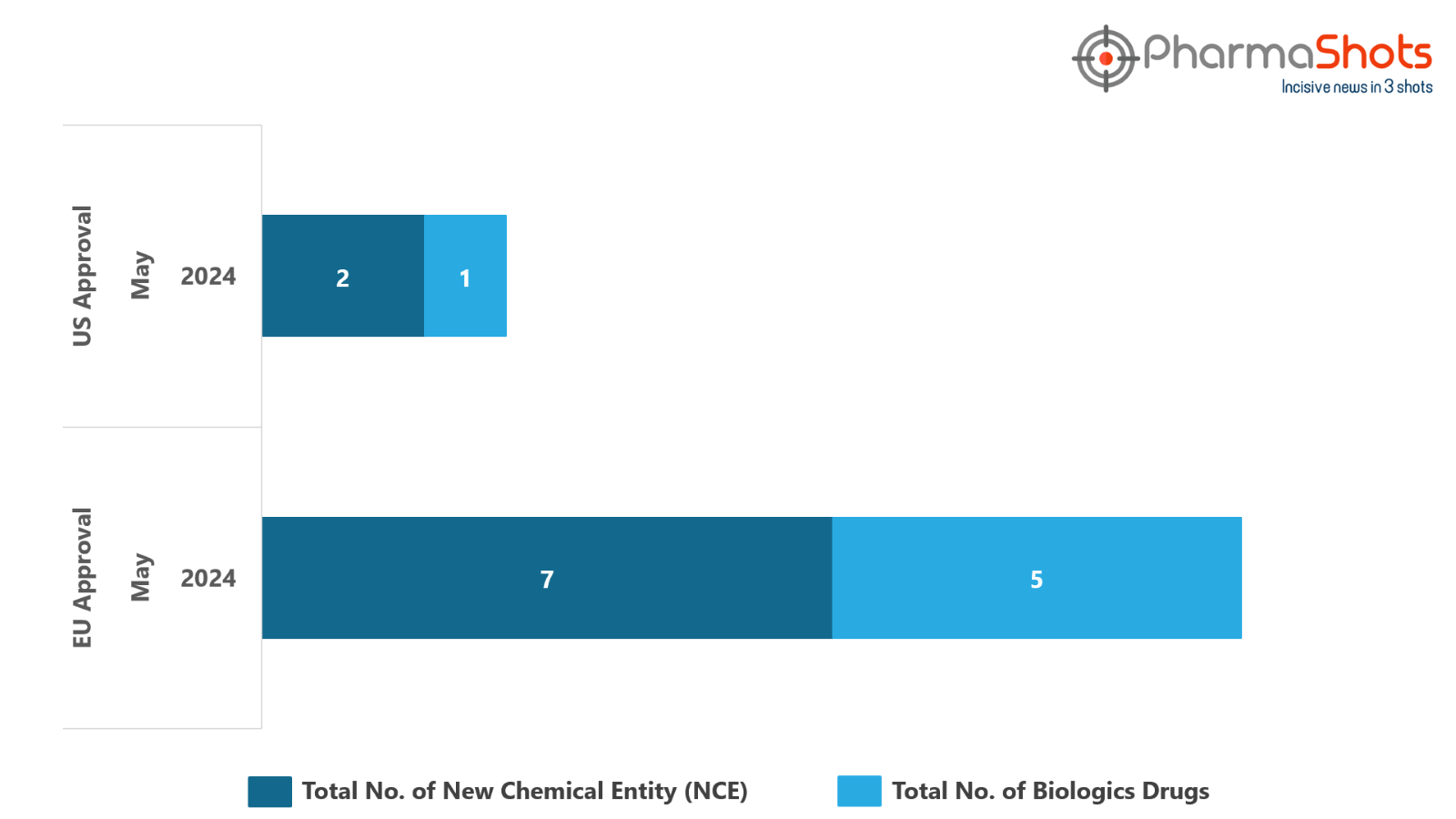
The EMA approved or granted Positive Opinions to 5 Biologics and 7 New Chemical Entities in May 2024, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were BMS’ Opdivo + cisplatin & gemcitabine to treat Urothelial Carcinoma and Takeda’s ADAMTS13 for the treatment cTTP
-
PharmaShots has compiled a list of 8 drugs that were approved or have been granted positive opinion by the EC or EMA’s CHMP, respectively
1. BMS’ Opdivo Plus Cisplatin and Gemcitabine Gain EC’s Approval for Treating Urothelial Carcinoma
Product Name: Opdivo
Active ingredient: Nivolumab
Company: BMS
Date: May 29, 2024
Disease: Urothelial Carcinoma`
Shots:
-
The EC has approved Opdivo + cisplatin & gemcitabine as 1L treatment of unresectable or metastatic urothelial carcinoma (UC) adults, valid across the whole EU along with Iceland, Liechtenstein & Norway, based on the P-III (CheckMate -901) study
-
The P-III (CheckMate -901) study assessed Opdivo + Yervoy or Opdivo + cisplatin & gemcitabine followed by Opdivo alone vs SoC CT alone to treat unresectable or metastatic urothelial cancer patients (n=608)
-
The results, with a median follow-up of 33mos., depicted 22% OS with mOS of 21.7mos. vs 18.9mos.; 28% PFS with mPFS of 7.9mos. vs 7.6mos.; 57.6% (n=175) vs 43.1% (n=131) ORR with CR [22% (n=66) vs 12% (n=36)] and PR [36% (n=109) vs 31% (n=95)]. Results were highlighted at ESMO 2023
Product Name: Tagrisso
Active ingredient: Osimertinib
Company: AstraZeneca
Date: May 31, 2024
Disease: Non-Small Cell Lung Cancer
Shots:
-
The CHMP’s positive opinion of Tagrisso + pemetrexed & Pt-based CT as a 1L treatment of locally advanced (Stage IIIB-IIIC) or metastatic (Stage IV) EGFRm NSCLC with exon 19 deletions or exon 21 (L858R) mutations was based on P-III (FLAURA2) study
-
The P-III (FLAURA2) study assessed Tagrisso (80mg, oral, QD) + CT [pemetrexed (500mg/m^2) + cisplatin (75mg/m^2) or carboplatin (AUC5), Q3W] followed by Tagrisso with pemetrexed maintenance (Q3W) in the above indication among 557 patients
-
The study depicted 38% reduced disease progression or death risk & 25.5mos. vs 16.7mos. mPFS with immature OS data (41% maturity); though a trend was seen in OS benefit, its evaluation is underway. Data was published in the NEJM
Product Name: ADAMTS13
Active ingredient: rADAMTS13
Company: Takeda
Date: May 31, 2024
Disease: Congenital Thrombotic Thrombocytopenic Purpura
Shots:
-
The CHMP has granted a positive opinion to ADAMTS13 (rADAMTS13) for treating ADAMTS13 deficiency in cTTP children and adults
-
The positive opinion was based on the P-III study assessing the efficacy, PK, safety, and tolerability for the same. Data was published in The New England Journal of Medicine in May 2024
-
rADAMTS13 is a recombinant ADAMTS13 enzyme replacement therapy intended to treat cTTP. It is also being evaluating for iTTP in an ongoing P-IIb study
4. CStone Pharmaceuticals’ Cejemly Receives the CHMP’s Positive Opinion for Treating NSCLC
Product Name: Cejemly
Active ingredient: Sugemalimab
Company: CStone Pharmaceuticals
Date: May 31, 2024
Disease: Non-Small Cell Lung Cancer
Shots:
-
The CHMP has granted positive opinion to Cejemly (sugemalimab) + CT as a 1L treatment of metastatic NSCLC
-
The positive opinion was supported by the P-III (GEMSTONE-302) study, demonstrating improvement in PFS & OS with sugemalimab + CT vs PBO + CT among treatment-naïve stage IV NSCLC patients. Results were published in The Lancet Oncology & Nature Cancer as well as highlighted international academic conferences
-
The marketing application of the combination is under review with MHRA for the same indication and is under discussions with the EMA, MHRA & US FDA for other indications. Additionally, it is seeking collaborations for sugemalimab apart from a deal Ewopharma in Central Eastern Europe and Switzerland
Product Name: Livmarli
Active ingredient: Maralixibat
Company: Mirum Pharmaceuticals
Date: May 31, 2024
Disease: Progressive Familial Intrahepatic Cholestasis
Shots:
-
The CHMP has granted positive opinion to Livmarli (oral solution) for PFIC patients (age: ≥3mos.) along with COMP for maintaining ODD. EC’s decision is anticipated in Q3’24
-
The opinions were supported by the P-III (MARCH) trial involving 93 patients with PFIC types (PFIC1, PFIC2, PFIC3, PFIC4, PFIC6 & unidentified mutational status), showing decreased pruritus severity
-
In addition, the company has submitted an sNDA for Livmarli’s higher concentration formulation utilized in the MARCH trial, allowing label expansion among younger PFIC patients across the US. It will be introduced in H2’24
Product Name: Dupixent
Active ingredient: Dupilumab
Company: Sanofi & Regeneron
Date: May 31, 2024
Disease: Chronic Obstructive Pulmonary Disease
Shots:
-
The CHMP’s positive opinion of Dupixent as an add-on maintenance therapy for uncontrolled COPD patients with type 2 inflammation was based on P-II (BOREAS & NOTUS) studies assessing its safety & efficacy for the same
-
Studies met the 1EP depicting a 34% reduction in annualized moderate or severe acute COPD exacerbations, sustained lung function improvements & improved health-related QoL at 52wks. which was significant & nominal in the BOREAS & NOTUS studies, respectively, as evaluated by SGRQ
-
Additionally, Dupixent’s sBLA for the same indication has been accepted by the US FDA & received priority review with the decision expected on Sep 27, 2024. Other regulatory submissions are under review globally (incl. US & China)
7. AbbVie Reports the CHMP’s Positive Opinion of Skyrizi (Risankizumab) to Treat Ulcerative Colitis
Product Name: Skyrizi
Active ingredient: Risankizumab
Company: AbbVie
Date: May 31, 2024
Disease: Ulcerative Colitis
Shots:
-
The CHMP grants a positive opinion to Skyrizi for treating moderate to severely active UC in adults with inadequate or lost response and were intolerant to conventional or biologic therapy. Final decision is anticipated in Q3’24
-
The positive opinion was based on the P-III (INSPIRE) study, assessing Skyrizi (induction treatment: 1200mg, IV, 0,4 & 8wks.) in moderate to severely active UC patients, and P-III (COMMAND) study, evaluating Skyrizi (180mg/360mg, SC, another 52wks.) in patients who responded to induction treatment
-
The two studies met the 1EP of clinical remission & 2EPs of endoscopic improvement and histologic-endoscopic mucosal improvement and showed consistent safety profiles without any new risks
8. SIFI Reports the CHMP’s Positive Opinion of Akantior (Polihexanide) to Treat Acanthamoeba Keratitis
Product Name: Akantior
Active ingredient: Polihexanide
Company: SIFI
Date: May 31, 2024
Disease: Acanthamoeba Keratitis
Shots:
-
The CHMP grants a positive opinion to Akantior for treating acanthamoeba keratitis based on the P-I & P-III studies. The final decision is anticipated in Aug 2024, to be valid across the EU incl. Iceland, Liechtenstein & Norway
-
The P-I trial determined the effect of different polihexanide concentrations vs PBO among healthy individuals (n=90) and verified that polihexanide (0.8mg/ml) had no significant differences in AEs vs PBO
-
The P-III (Study 043/SI) trial of polihexanide (0.8mg/ml or 0.08%) in acanthamoeba keratitis adults & adolescents (n=135) showed 86.7% of clinical resolution rate with 4mos. median time to cure plus improved vision & QoL
Note:
According to the EMA’s May 2024 approval list, the following drugs were also approved; however, no PR was available:
-
Tevimbra
-
Eliquis
Following drugs received CHMP’s Opinion; however, no PR was available:
-
Durveqtix
-
GalliaPharm
Related Post: Insights+: EMA Marketing Authorization of New Drugs in April 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














